Introduction
Nuclear physics is the field of study that concerns the atomic nuclei, and some understanding of nuclear physics is needed to grasp the problems related to nuclear weapons. If you remember your chemistry or physics classes from school, you will know that the atom is the smallest part of an element, and an atom consists of a nucleus and an electron shell. There are two types of particles (of almost equal mass) that constitute the atomic nuclei: the proton, which is positively charged, and the neutron, which is electrically neutral. The number of protons defines a specific element, i.e. 6 protons make up the element carbon (chemical symbol C), and 92 protons make up the element uranium (chemical symbol U). All the elements, ordered based on increasing proton numbers, are commonly presented in the periodic table of elements.
In addition to the protons, all nuclei with at least 2 protons also contain neutrons, and the number of neutrons can vary in atoms of the same element; these varieties are called isotopes. The isotopes are named after the total number of protons and neutrons, so for example carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons and 8 neutrons. Isotopes of the same chemical element have the same chemical properties, but differ in mass and have different nuclear properties, such as radioactivity. One example is heavy water, which is water containing not normal hydrogen (with only 1 proton) but heavy hydrogen, so called deuterium (with 1 proton and 1 neutron).
For the heaviest element that exists on Earth – uranium – the two isotopes that occur naturally in more than trace amounts are uranium-235 (92 protons, 143 neutrons) and uranium-238 (92 protons, 146 neutrons). Uranium is interesting in this context because it is the starting point for creating nuclear weapons. The isotope of most interest in this respect is uranium-235. In naturally occurring uranium, only 0.7 percent (mass) is uranium-235, while 97.3 percent is uranium-238. The process of increasing the fraction of uranium-235 in a uranium sample is called enrichment. Enrichment can be carried out with various technologies, but the most common is the centrifuge technique which separates the isotopes based on their mass difference. First, a uranium gas is created, and when it is spun rapidly in a centrifuge drum, isotopes of different masses will follow different trajectories and can thus be separated.
Fission
Influences on critical mass
How can the critical mass of nuclear material be modified according to changes in density, volume, and the addition of neutron reflector material?
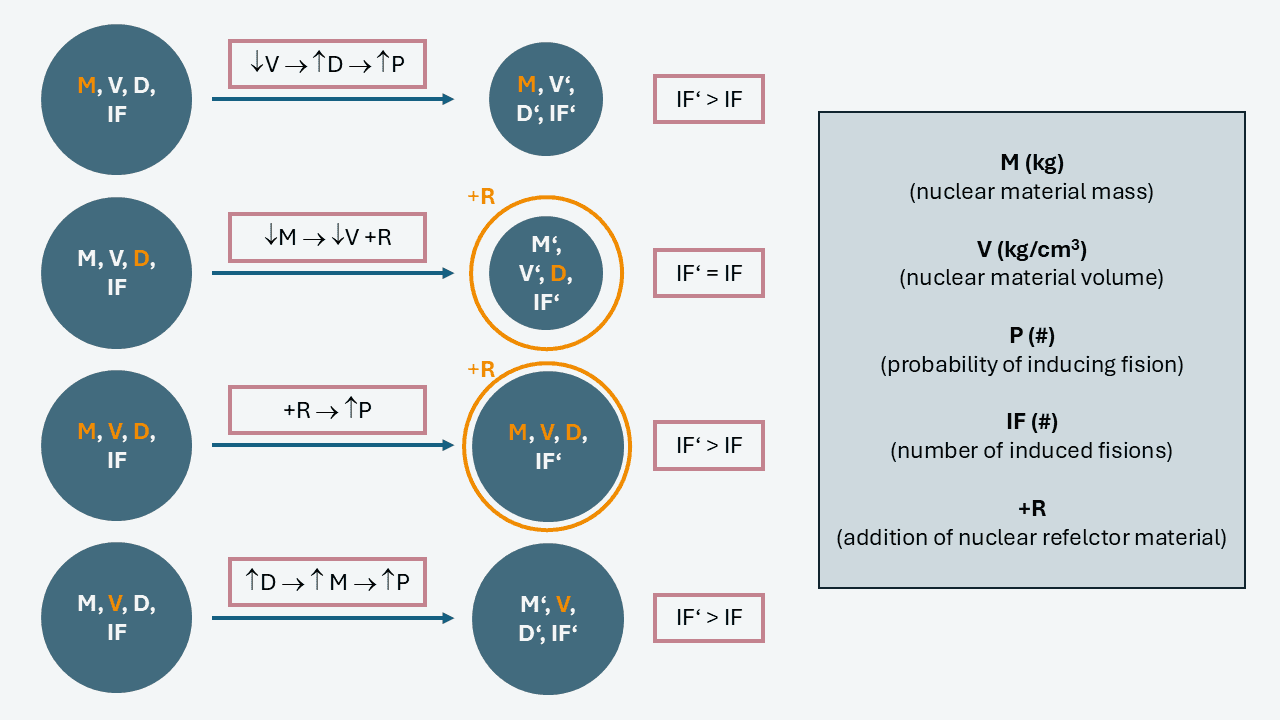
A) If the volume of nuclear material is decreased by compressing it, its density will increase, increasing the probability of inducing fission reactions for the same mass.
B) If the mass of nuclear material is decreased, keeping its density unchanged, the same level of induced fissions can be kept by adding a neutron material reflector around it.
C) If the volume, mass, and density of the nuclear material are unchangeable, the probability of inducing fission can be increased by the addition of neutron reflector material around it.
D) If the density of nuclear material is increased without changing its volume, the mass of nuclear material will increase along with the probability of inducing fission.
Radioactivity
Another topic of interest is radioactivity. Radioactivity occurs naturally and is a spontaneous process where a nucleus decays by the emission of ionising radiation. Of the elements in the periodic table, all have several isotopes and most have both stable and radioactive ones. The conditions within the nuclear structure that determine whether a nucleus is stable or radioactive are complex, but one of the factors that play a role is the relative number of protons and neutrons. In general, light elements have stable isotopes with equal or nearly equal numbers of protons and neutrons, but in heavy elements, there are substantially more neutrons needed for the formation of a stable nucleus. Heavy nuclei have more neutrons than protons, and this is why, when a heavy (uranium) nucleus splits into two lighter ones, the resulting fission products are left with too many neutrons to remain stable. This is the basis for the formation of highly radioactive products from fission.
A radioactive material decays with a unique half-life, i.e. the time it takes for half the radioactive nuclei in a sample to decay. After the decay, that particular nucleus has been transformed into an isotope of another chemical element, which can be stable or unstable (radioactive). If the resulting nucleus is stable, the decay stops here, but if it is unstable, the new nucleus will also decay according to its half-life. In some cases, there are long decay chains with many intermediate steps, until the final stable nucleus is reached.
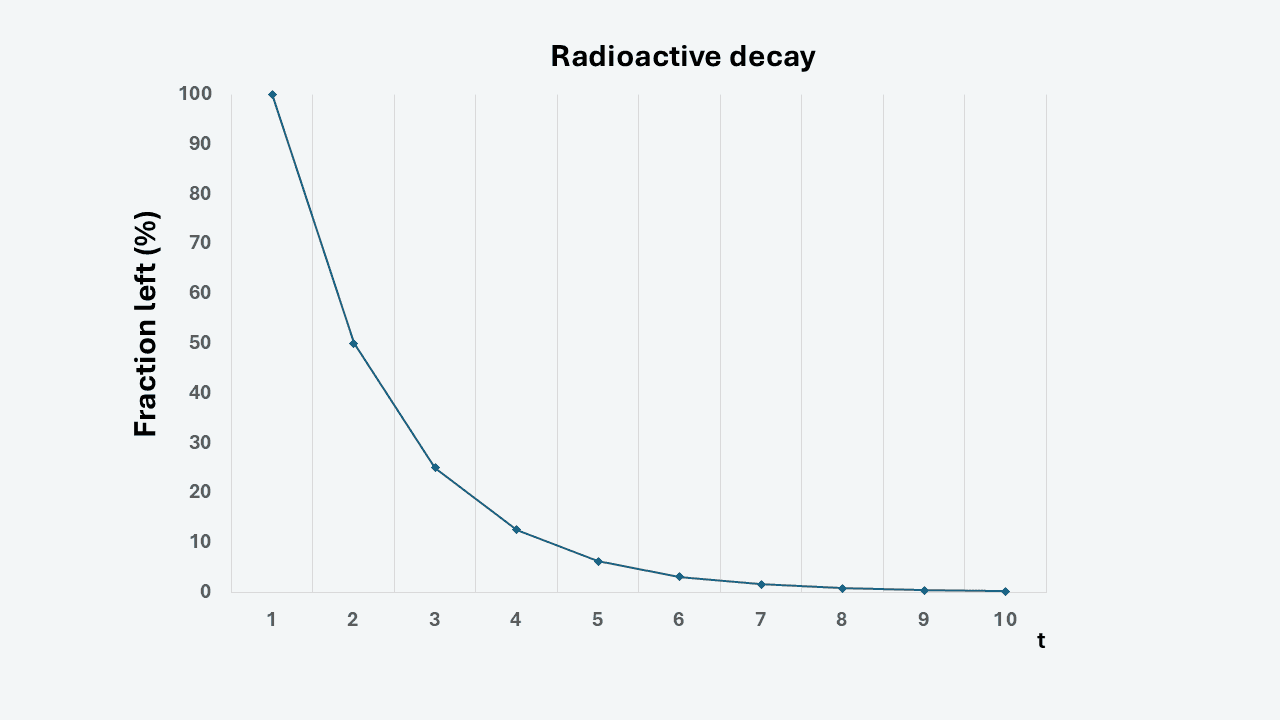
The decay curve is a negative exponential function (Figure: half-life) with the properties that after one half-life, 50 percent of the original amount remains, after two half-lives, 25 percent remains etc. After ten half-lives, less than 0.1 percent remains and this is generally regarded as the time it takes for a radioactive substance to be gone. Radioactive fission products are usually the starting points of long decay chains with several steps to the final stable element. The half-lives of these radioactive elements range from fractions of seconds to decades. Some isotopes that often are mentioned in nuclear technology applications are iodine-131 (half-life: 8 days) and caesium-137 (half-life: 30 years).
Radioactive decay comes in three forms – alpha, beta and gamma decay – with radioactive isotopes normally decaying either via alpha or beta decay, and the gamma decay following shortly after the initial alpha or beta. Alpha, beta, gamma and neutron radiation is all ionising, meaning it has enough energy to ionise atoms and molecules.
Alpha decay happens in isotopes of many heavy elements, such as radon, uranium and plutonium, and in the decay, an alpha particle is released. This is a heavy, charged particle that is, in fact, a helium nucleus consisting of 2 protons and 2 neutrons. Such a particle travels only short distances in air and even shorter in a denser material. Alpha particles can typically be stopped by skin or by a thin piece of paper.
Most radioactive elements naturally present on Earth, as well as most fission products, decay via beta decay. The beta decay releases an electron and a neutrino, but only the electron interacts with matter and is regarded as ionising radiation. Being a much smaller particle, albeit charged, the beta particle has a longer range than an alpha particle and requires thicker material to be stopped.
The gamma particle that is released, often together with an alpha or beta particle, is a normal photon but with high energy. Gamma rays are similar to X-rays, but originate in an atomic nucleus, rather than in an atomic electron shell which is the origin of an X-ray. These particles have no mass and no charge, making them highly penetrable. They can travel a long way (at the speed of light) and require a dense material such as lead to be stopped.
The release of neutrons is particularly common for fission events but not a common natural form of radiation. Neutrons have mass, but no charge, and they can interact only with other atomic nuclei, not with electron shells.